Structural insights into mechanism and specificity of the plant protein O-fucosyltransferase SPINDLY
Authors:Li Zhu, Xiting Wei, Jianming Cong, Jing Zou, Lihao Wan & Shutong Xu
Nature Communications.02 Dec 2022.
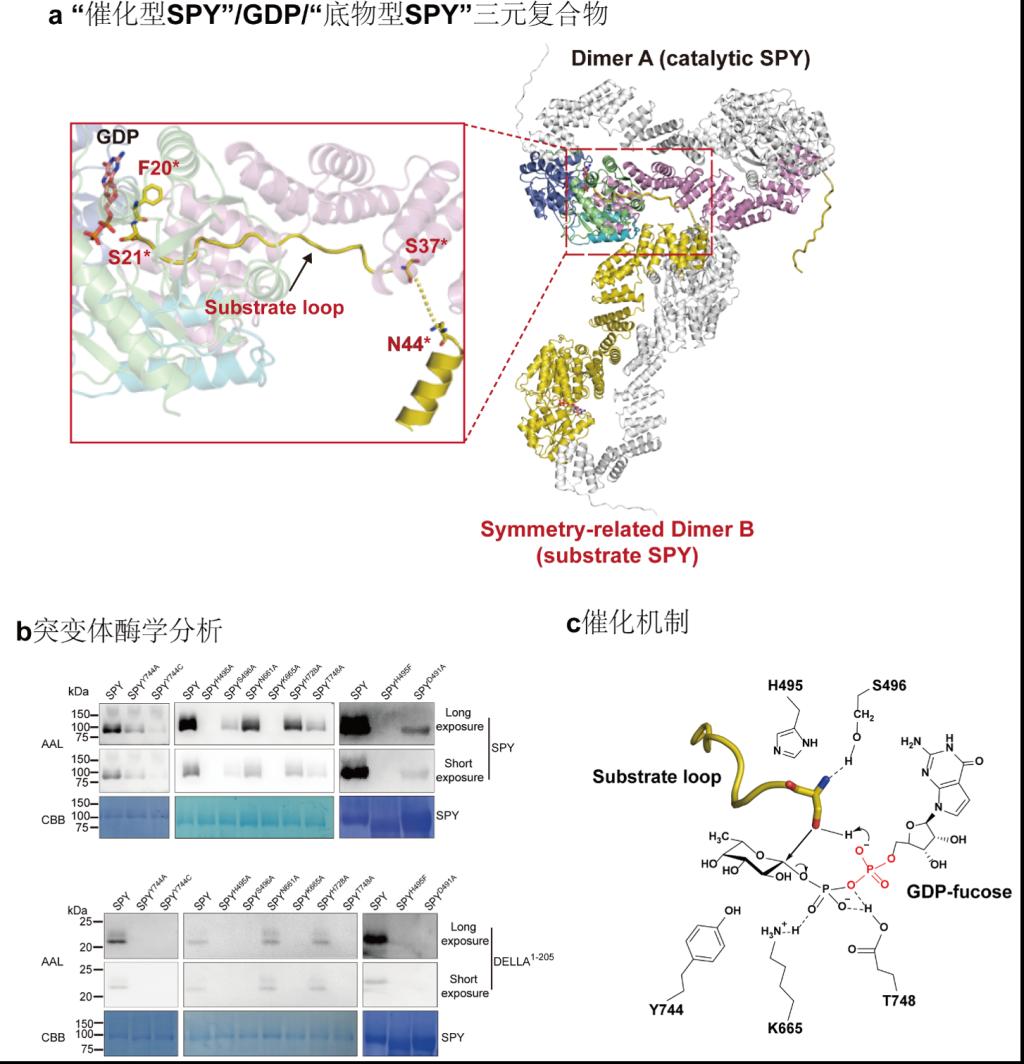
Abstract: Arabidopsis glycosyltransferase family 41 (GT41)protein SPINDLY (SPY) plays pleiotropic roles in plant development.Despite theamino acid sequence is similar to human O-GlcNAc transferase, Arabidopsis SPYhas been identified as a novel nucleocytoplasmic protein O-fucosyltransferase.SPY-like proteins extensively exist in diverse organisms, indicating thatO-fucosylation by SPY is a common way to regulate intracellular proteinfunctions. However, the details of how SPY recognizes and glycosylatessubstrates are unknown. Here, we present a crystal structure of Arabidopsis SPY/GDPcomplex at 2.85 Å resolution. SPY adopts a head-to-tail dimer.Strikingly, the conformation of a ‘catalyticSPY’/GDP/‘substrateSPY’ complex formed by two symmetry-related SPY dimers is captured in thecrystal lattice. The structure together with mutagenesis and enzymatic datademonstrate SPY can fucosylate itself and SPY’s self-fucosylation regionnegatively regulates its enzyme activity, reveal SPY’s substrate recognitionand enzyme mechanism, and provide insights into the glycan donor substrateselection in GT41 proteins.
Full article:
https://www.nature.com/articles/s41467-022-35234-0#Ack1